
Cascade Metrix (CMX) is a medical technology company working within a new paradigm in critical care. The company will introduce an Automated Multiplex Analyzer ["AutoPlexer"] that will revolutionize the bedside testing of hospitalized patients whose care and recovery is dependent on repetitive, around-the-clock measurements of critical blood parameters. This innovative and proprietary ex-vivo blood analysis system is first being applied to the automated multiplex measurement of glucose, lactate, hemoglobin and oxygen saturation in critically ill patients and will over time expand to include multiple other biomarkers of interest.
Latest Update: CMX has been awarded a highly competitive SBIR Phase II grant [2R44GM146498-02; A Multiplex analyzer for Sepsis and Beyond; PI Kislaya Kunjan, PhD; 2025-28) from National Institutes of Health; RePORTER.NIH.gov Portal]. This was following the successful completion of the SBIR Phase 1 grant deliverables. In addition to this, Elevate Ventures had provided additional non-dilutive matching funds.
CMX has completed the prototypical development and preclinial validation of the AutoPlexer solution to support early diagnosis and treatment of sepsis. Efforts are currently underway for inpatient trials ahead of regulatory filings.
CMX is seeking investment as well as partnership opportunities with firms in the medical technology, health systems and related areas. Please contact
Cascade Metrix [CMX] in partnership with Purdue University’s Pharmacology Testing Facility (West Lafayette, IN) conducted a 3-day pilot study on two pigs. The study purpose was to inform the design and assess performance of the ex-vivo automated glucose monitoring system (AGMS) prototype developed by CMX (see Figure 1). At user defined measurement intervals, the device withdraws blood from the subject’s vascular line for measurement across a flow-through glucose sensor followed by complete reinfusion of the blood. Before each measurement the system performs an automated calibration. The study was conducted from March 21-24, 2016.
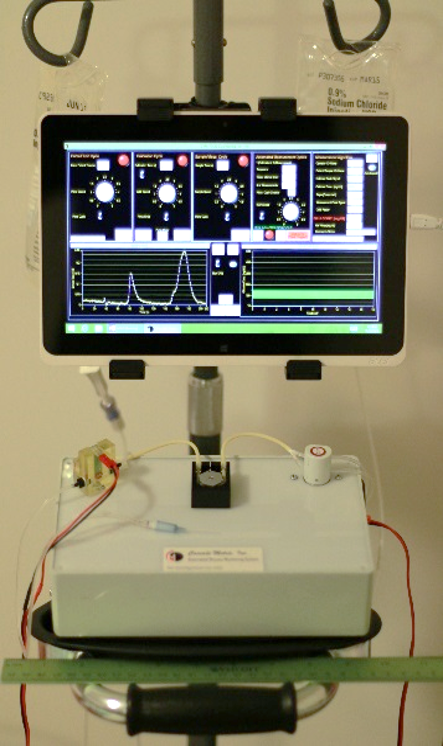
Fig 1: AGMS Prototype
Trial Set-Up: Two young pigs [2 months old, each weighing 30 kg] were tethered to two devices placed adjacent to movement-responsive caging stations (Figure 1) from BASI (West Lafayette, IN).

Figure 2: Trial Set-up at the Purdue Pharmacology Testing Facility
Central venous catheters were surgically placed in the jugular veins of the pigs. Each pig was tethered to a device using a 10 feet long micro-bore tubing. There were 2 devices [Dev1 and Dev2]. For Dev1, the flow-through glucose sensor was placed proximal to the subject secured to the tubing, while for Dev2, the flow-through sensor was placed proximal to the device enclosure. There were 2 fluid bags filled with non-heparinized buffered saline. One bag was used for rinsing, while the other was for calibration [100 mg/dl reference]. Both the devices were controlled from a single Tablet PC. The study period was 72 hours.
Results: Dev1 was able to perform the automated sampling routine for the entire 3 day period. Dev2 on the other hand encountered sluggish blood flow on Day 2, likely due to the long blood flow path of 10 ft. for each measurement cycle. The sluggish flow was resolved through a manual flush (thereby opening up the thrombus sheath) and by bring the flow-through sensor proximal to the subject (thereby reducing the blood volume required to be drawn out of the subject).
On day 3, an intravenous GTT (Glucose Tolerance Test) study was done on the pigs. 30ml of D50W was infused into the pig’s central line and multiple reference measurement were performed during the study [Figure 3].
Figure 3: Infusion of Dextrose for the Glucose Tolerance Study
The results from the GTT study are shown in figure 4 and figure 5 below. The glucose as measured by the device is shown in BLUE, while the reference glucose measurements are marked in RED.

Figure 4: IV Glucose Tolerance Study on Dev1
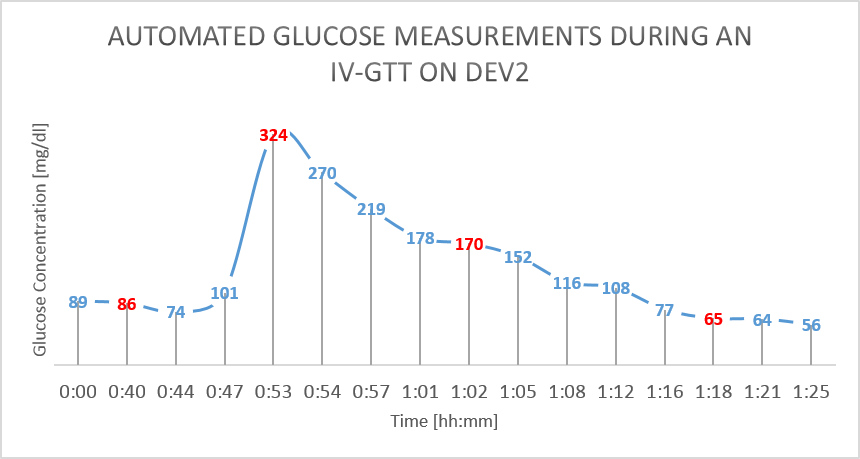
Figure 5: IV Glucose Tolerance Study on Dev2
Conclusion and Next Steps: The study demonstrated the reliability of automated sampling and glucose sensing in tethered pigs over a 3-day period without the need for heparin infusion. It is imperative that the blood volume drawn out of the subject is minimized by securing the flow-through sensor as close to the subject as possible. This will minimize the risk of thrombus sheath formation at the catheter tip. Additional studies on small animals are indicated after implementing the device design changes identified from this study, including expanding the range of measured analytes.
Need for Inpatient Glycemic Control
The intensive care units have a very high prevalence of hyperglycemia (> 40%). Additionally, hypoglycemia and glycemic variability are well recognized risk factors. It was not until 2001 that a “landmark” study by Berghe et.al. established the need for tight glycemic control [i.e., normalizing glucose through intensive insulin therapy] in ICUs. Significant reduction in mortality, morbidity (due to sepsis, renal failure, blood transfusion), and length of stay (LoS) savings were observed. Financial savings were estimated at $3,345/patient due to reduced LoS and avoided interventions. However despite protocols calling for tight glycemic control, there was poor compliance due to the existing labor-intensive and sub-par accuracy measurement methods which were typically based on capillary fingerstick sampling and meter/strips. The US Medicare system instituted “no-pay” guidelines for complications that arise from poor glucose control.
Challenges with Sepsis Management
Sepsis (defined as a dysregulated host response to infection that causes life-threatening organ dysfunction) is typically caused by blood-borne infections, which may be bacterial, viral or otherwise. Specifically, upon the earliest suspicion of Sepsis, the blood lactate levels should be assessed as part of the Surviving Sepsis Protocol, which helps to rule out or identify the root cause of a lactate elevation. If lactate is elevated (> 2 mmol/L), it can be due to elevated glucose values (Glucose monitoring would address this), internal bleeding (Hemoglobin would address this), need for resuscitating fluids (Central Venous Oxygen Saturation, ScvO2 would address this), organ failure (rare, but heart monitor can address), and blood infection. The knowledge of the "big four" analytes enables the "race" to administer triple anti-biotic therapy, which is today delayed considerably.
Existing Solutions
Currently, blood glucose monitoring is done using labor intensive fingerstick based meters/strips offered by companies like Roche Diagnostics, Nova Biomedical, and Abbott. Multi-analyte measurements are done using products like the i-Stat which uses single use cartridges or blood gas analyzers based on multi-use cartridges from companies like Radiometer (Danaher), Instrumentation Laboratories and Siemens Healthineers. Not only are blood gas analyzers labor intensive, they also are associated with long turn around times, have high cost, expose the care giver to infectious blood and above all result in substantial blood loss to the patient. Central venous oxygen saturation measurements utilize dedicated central venous catheter based fiber-optic systems from companies like Edwards Lifesciences and ICU Medical, but their use remains restricted.
Thus the CMX 4-plex solution provides a one of its kind system for early glycemic management and early Sepsis diagnosis and treatment that is associated with significant mortality and morbidity reductions.
